I’m re-sexifying the top of this post since it’s so long and I want people to get these key take-aways (read on for details on each one):
- Expect clinical trial participants to share news with their networks. Plan accordingly.
- Make your information attractive and share-able where people already are, on Facebook, Tumblr, Pinterest, etc.
- Find your local Lady Gaga.
- New Coke is a model for what not to do when engaging a community.
- “Re-sexify” a repetitive message because there are some things you can never say enough.
- Integrating a new social media tool is like having a new employee, it’s that much of a productivity hit.
I participated in the National Institutes of Health’s HIV/AIDS Clinical Trials Networks Communications Symposium on May 9, 2013, sharing Pew Research data about internet and cell phone use across the globe and, in particular, how people in the U.S. gather and share health information, online and offline.
My notes from the discussion are below, but I make no claims about them other than I wrote down stuff I was curious about or thought would have universal application:
“The moment you tell one participant, the race is on to tell the rest. They want to hear the news from clinicians.”
The first presentation was a riveting tick-tock of how the HVTN 505 HIV Vaccine Regimen Study announced that they were ending the administration of injections. “Dear Participant” letters were sent out and almost immediately posted on personal blogs, Twitter, and the clinical trial wiki. Unfortunately the wiki update by a participant contained an error — “the joy and the discomfort of openness.” There was no “media” leak in the traditional sense, but there was a wiki leak (yes, that got a laugh), so they stuck with the planned schedule and did not rush out the press announcement because it was contained within the community.
Lesson: Make rapid response to participants part of the study protocol. Expect people to share with their networks. Monitor every channel and have multiple contingency plans.
One voice, many inflections.
Amy Ragsdale, the meeting organizer, then introduced me and it was like getting on a high-speed elevator to the top of the Empire State Building: we went from a street-level case study to a global view of the health communications landscape. Amy came up with the title for my talk, which I love since it captures the main idea of public health messaging: one voice (ie, the facts, the best science available) many inflections (help and encourage people to share those facts in their own ways, with their communities):
[slideshare id=20820734&doc=pewresearchinternethealthglobalmay2013-130508152006-phpapp01]
I think one of the key take-aways from my talk, based on the discussion, was that HIV clinical trial communications about treatment should still focus on outreach to clinicians since Pew Research data show that MDs and RNs are still the #1 source for that type of information. But communications experts can also take heart in the fact that they can use the “many megaphones” approach when it comes to outreach, education, trial recruitment, and other activities.
Amy shared an important distinction during the meeting and captured it in a follow-up email:
The HIV prevention world confronts different challenges and opportunities than the treatment world. In prevention, we reach out to those who generally don’t think of themselves at risk and don’t have the immediate need of clinical care. Health messaging to “healthy” people requires a different approach – especially when engaging them around recruitment into a trial.
That is a significant challenge that I’d love to crowd-source a bit — if you have ideas or amplifications to share, please do so in the comments below.
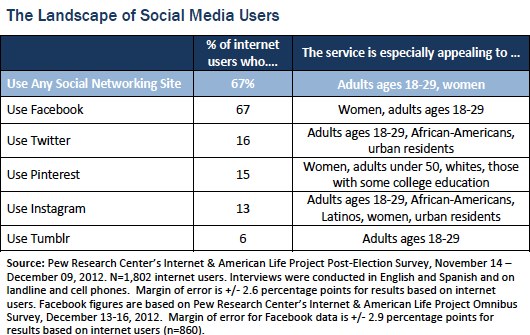
A first step is to be sure that your information is share-able on the platforms your target audience is using. Here’s a table I should have included in my slides, but didn’t:
(Note: this is why I love social media — the conversation is never over.)
Meeting participants were disappointed that I didn’t have data from East or South Africa to share, but my colleagues in the Pew Global Attitudes Project tell me that we will have that data soon. Meantime, we heard stories about fieldworkers in Zimbabwe, Malawi, Zambia and other countries trading Facebook messages, not email, so there are indications that the social networking wave affects those countries’ health communications, too.
I wish that I had emphasized the “listen more than ask” aspect of the new media (and new research) landscape, but I think the other presenters hit those points better than I could have, anyway. This is a group of people with deep ties to both research communities and the patient/caregiver communities.
Joe Camel used to own the airwaves and we had few tools to combat his hold on kids. That’s no longer true.
That was a comment from one of the participants, along with her observation that there are now many voices, many airwaves, many megaphones. A tweet from Lady Gaga — or the Lady Gaga equivalent in a micro-community — can be influential. Find the Lady Gagas in your community. Leverage what your community is already doing, for example, the Asian & Pacific Islander American Health Forum created a fun video about open enrollment that other organizations are now using:
However, it’s difficult to predict what will be hit. For example, a rectal microbicide how-to video may be a dud in one country, like Peru, but a hit in another, like Thailand. Is it because of faster internet connections? Higher interest? Better outreach on-site? It’s unknown, so the current strategy is to continue to generate accurate, compelling messages and test them in different markets, in different ways.
Advertising about a clinical trial is considered recruitment, which is considered informed consent.
There was a technical discussion about clinical trial recruitment and when to involve an Institutional Review Board (IRB). The bright-line rule: no “ask” means no need for IRB approval. But social media introduces complexity: currently, a piece of paper triggers IRB review. If you read all the contents of that piece of paper to someone but don’t hand it to them, no IRB approval is needed.
New Coke is a model for understanding how people will integrate a clinical trial protocol into their lives.
Coca-Cola missed that there is a culture of Coke drinkers, just as clinical trial investigators can sometimes miss the culture of sexual practices and preferences in the target population. It wasn’t that the product was so bad, but rather, the company did not respect their audience enough to talk with them first and understand how they might need to change the protocol.
Lesson: Engage the community early. Have community members on the research team.
Community-level messages about HIV clinical trials should be simple, straightforward, and sexy.
It is a significant challenge to balance enthusiasm, expectations, and outcomes. How to communicate about a clinical trial that hasn’t cured HIV, or even solved a problem, but at least has moved the field forward? Maybe: “It is a good thing the trial was stopped because it means we learned something and are ready to share.” Redefine failure, help people understand basic research literacy. Sell, but don’t oversell, hope. “Re-sexify” a repetitive message because there are some things you can never say enough.
Some of these lessons can be translated to press strategy. Reporters who want to cover a clinical trial as a “win” or a “loss” need to be educated about the scientific process of discovery.
“Integrating a new social media tool is like having a new employee, it’s that much of a productivity hit.”
This was a quote from the discussion at the end of the day, after we heard a smart presentation about evaluating the return communications investments of social tools along the lines of site recruitment, scientific publications, lay press, public health interest, etc.
The “something shiny” syndrome is familiar to everyone, which is why I appreciated the discussion about how to leverage existing content and make it easy to share. You don’t have to be on every platform; you want your information to be available there. I likened it to developing new muscles and using them in different ways — the blog muscle becomes the Tumblr muscle becomes the Whatever’s-Next muscle. Make it easy to share. In other words: one voice, many inflections.
All in all, it was a day devoted to working on stuff that matters and I was honored to contribute.
I wish I could have been there! My comment is based on experience with clinical trials in oncology, but I think it is relevant to HIV trials as well. We often underestimate how much science patients and trial participants can understand. Not only does this mean we can have thoughtful, open discussions about the details of a given trial, it also means that patients can and should participate more in the *design* of trials. How might trials be different if patients were more explicitly involved in their design? How much easier would it be to enroll patients in trials more quickly if the patient community knew that other patients were involved in the trial design?
Yes! This is why I wrote this post, hoping that we could start a cross-disciplinary conversation. Thank you.
I’ve spent the last five years in and around health tech and software development. I think any time you put the end user into the design and testing phases, you are likely to improve quality of the product and expected outcomes with fewer iterations. Yes, you’ll hit snags along the way, but if we work together trial researchers may learn that their assumptions with regards to what a patient may tolerate could be completely wrong. While my ulcerative colitis doesn’t not require the cocktail of medication often associated with HIV, I have had to go through a lot of trial and error to find the best therapeutic solution for me (which resulted in a sicker and therefore grumpier me for 6-8 months). In this instance, the next option of medicine may have relieved my ibd symptoms, but the risk of leukemia was far too high for a young woman of reproductive age. I lucked out when I conducted my own research and found studies that show that probiotics can functionally “cure” the disease. Even if it isn’t trial design, having a provider who is open to an engaged patient can make all the difference in outcomes. I’m on the mend now and hope I stay on the good health side for a long time.
So the HIV Vaccine Trials Network (HVTN) has Community Advisory Boards (CABs) which provide community input into study design and local procedures. Each trial site has a CAB. Here is the link for more information about HVTN CABs http://www.hvtn.org/community/cab.html
Susannah Can you provide more information about this segment:
Advertising about a clinical trial is considered recruitment, which is considered informed consent.
There was a technical discussion about clinical trial recruitment and when to involve an Institutional Review Board (IRB). The bright-line rule: no “ask” means no need for IRB approval. But social media introduces complexity: currently, a piece of paper triggers IRB review. If you read all the contents of that piece of paper to someone but don’t hand it to them, no IRB approval is needed.
As someone interested in the use of social media for research recruitment, can you give more specifics about when IRB approval is or isn’t needed?
Yes, I’m going to try to get one of the experts from the symposium to respond. I have little experience with IRBs so my notes are not detailed enough to shed much more light.
Thank you!
So there was no bright line on this (hence the title: the gray zone…) but what was presented suggested that the issue for debate was when information was provided such as in a news piece, versus using this information to solicit participation in a trial. The example given was that if an investigator/clinician read a news article describing a trial to someone they were providing information only. However, once that news article was given to that person by the investigator, there was now an implicit “ask” and would need IRB approval similar to any solicitation used in a clinical trial. If you are using social media to solicit participation in a trial, IRB approval would be required. If an informational piece was written, for example a blog post about a trial, then this would not necessitate approval.
Thank you! That is very helpful!
Thanks for presenting, Susannah. I thoroughly enjoyed it.
I focus more on the method, while my colleague crafts the message. So I was also struck by the need to be mindful of the technical side of knowing your audience, with regards to international outreach and recruitment. Knowing that the average connection speed in Malawi (<1 Mbps) versus Thailand (11 Mbps) can dictate the efficacy of video as an outreach tool. Or knowing that Brazil and India also use Orkut heavily can cause you to rethink which channel to use for your message.
I'm looking forward to getting more data from you on Africa so we can continue to fine tune our efforts.
To be useful to a group like this symposium gathered, to a person doing the work you’re doing… that’s better than anything. It’s what fuels the Pew Research Center to continue our work. Thank you.
Thanks, Susannah – For me, at the end of the day, the power of spreading information and storytelling is the most important thing. Identity (linked to branding), storytelling (marketing/promotion), trust (transparency and love), and community (social networks – both on and offline) are timeless best practices.
But we still need the tools: online, mobile, advertising. Thanks for working to ensure that we know which one can actually make a difference! For evaluating whether or not you are reaching people online, here’s one of my favorite catalogues: http://bigthink.com/digital-politics/a-catalogue-of-social-media-and-related-tools
Thanks Susannah for another insightful piece. For the past decade, I have worked in technology transfer and innovation management and just as in clinical trials, the communication is often one of the most important and poorly understood pieces. Truly understanding the audience is so critical or so much time can be spent without reaching the objective. Organizations often think in a one-size-fits-all approach, but that needs to change. I have heard from clients that they are concerned with cost of adapting the messaging (both message and medium) for different target populations, but that incremental increase in cost and time reaps significantly higher yields on the back end. I appreciate your approach of sharing lessons and looking for cross-disciplinary conversation. Thank you!
Update: my colleagues at the Pew Research Center have released a new study that will be useful to people following this thread:
Emerging Nations Embrace Internet, Mobile Technology
Cell Phones Nearly Ubiquitous in Many Countries
Feb. 13, 2014
Countries surveyed:
Argentina
Bolivia
Brazil
Chile
China
Egypt
El Salvador
Ghana
Indonesia
Jordan
Kenya
Lebanon
Malaysia
Mexico
Nigeria
Pakistan
Philippines
Russia
Senegal
South Africa
Tunisia
Turkey
Uganda
Venezuela